Runaway Biology: A Call for Conscientious Genome Editing with CRISPR
By Søren Hough
Volume 23, number 3, Bio-Politics

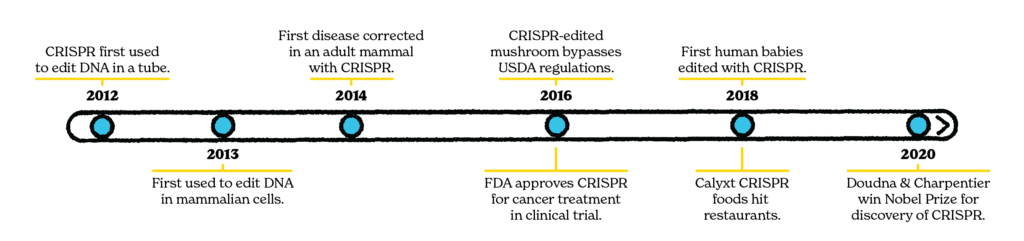
By 2018, it was clear that CRISPR had spun out of control. In the United States, one biotech company used spurious public health claims to get CRISPR-modified foods onto people’s dinner plates. Not long after, a world-shaking report revealed that CRISPR had also been used without formal approval in China to edit the DNA of two baby girls, Lulu and Nana. As scientists and governments methodically deliberate on the best way to regulate CRISPR’s use in society, those seeking fame and fortune plow ahead heedless of the consequences. CRISPR, now a Nobel Prize-winning technology, is a permanent fixture in biological research and clinical medicine. We must take its dire ethical implications, from changing the food we eat to altering human evolution, more seriously.
Genetic Reductionism and CRISPR: Dire Bedfellows
When Drs. Jennifer Doudna and Emanuelle Charpentier were awarded the Nobel Prize in Chemistry for their work on CRISPR, the Nobel committee framed their discovery as a tool for rewriting the “code of life” DNA.1 Describing DNA as the “code of life” is a common trope, and one that we should disabuse ourselves of. Though our psychological, social, and biological development are certainly affected by genetics, DNA alone cannot capture the whole picture. Referring to DNA as the code of life, a rigid blueprint for who we are, implies that we are the mere products of a series of chemical letters inherited from our parents. This is known as genetic reductionism — the idea that our very identity boils neatly down to a genetic code. Genetic reductionism and its cousin, biological determinism, are dangerous canards and potentially deadly in the age of genome editing.2
Taken to its logical conclusion, genetic reductionism pins everything from social status to intelligence on hereditary fate, ignoring and excusing the sociopolitical context that underlies these categories. We know, for example, that social determinants such as education and economic stability are crucial predictors of long-term health.3 Take suicide, one of the leading causes of mortality in America.4 Many researchers are striving to understand the genetic underpinning of suicidal behavior.5 From a genetic reductionist point of view, suicidality is the mere result of genetic mutation. Yet we also know that societal change like raising the minimum wage has a demonstrable effect on lowering suicide rates in America among individuals with a high school education or less.6 This kind of nuance is lost in a genetic reductionist approach that ignores evidence-based social solutions in favor of biological intervention.
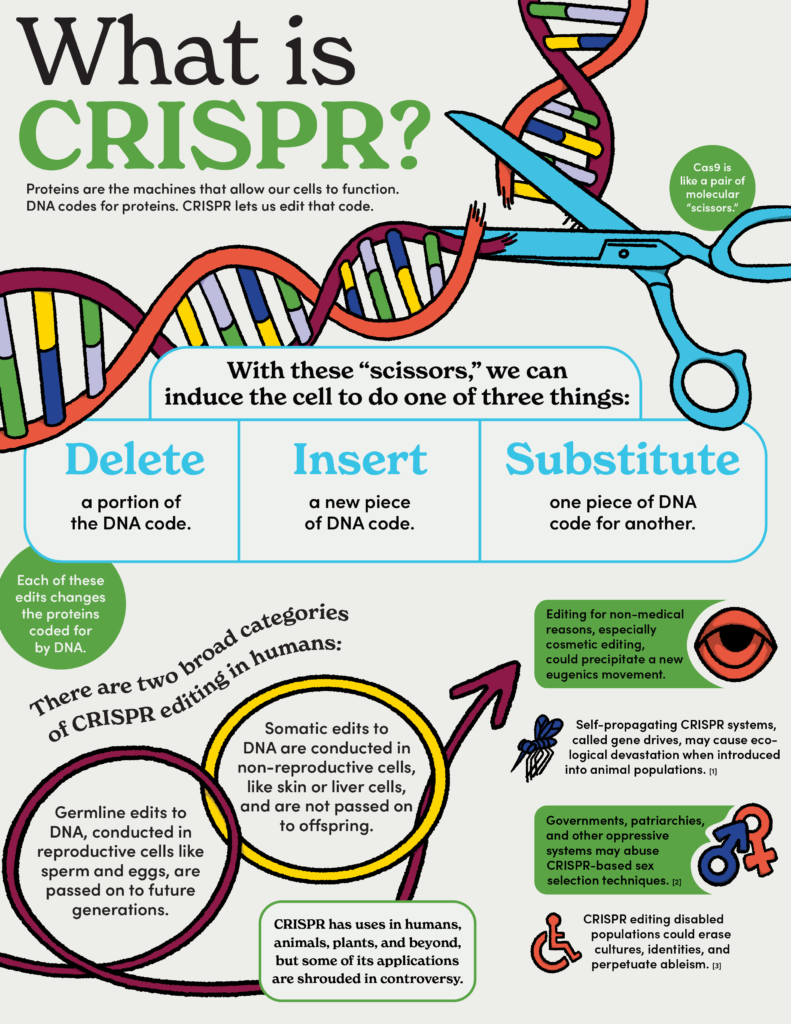
If society adopts a genetic reductionist outlook, it may lead governments and scientists to try to solve social problems with CRISPR rather than public policy. A Carter administration report on the matter, entitled “Splicing Life,” flagged this possibility, saying, “if genetically engineered changes ever become relatively easy to make, there may be a tendency to identify what are in fact social problems as genetic deficiencies of individuals or to assume that the appropriate solution to a given problem, whether social or individual, is genetic manipulation.”7 This is the eventuality warned against in the 1970s and 1980s by feminist biologists like Dr. Ruth Hubbard, who argued that people are “more than the sum of their parts” and that genetic reductionism is an “oversimplification” of biology.8 Hubbard indicated that addressing economic and social inequities in public health should be prioritized as alternatives to genome modifying technologies.9
CRISPR Is Not a Panacea for Social Ills
There is a strong current in global capitalism and political hierarchies, often intertwined with one another, that seeks to apply “techno-fixes” to the world’s problems. CRISPR presents just such an opportunity: for proponents of this worldview like Bill Gates, CRISPR is a chance to attack old problems like the spread of malaria from a whole new, possibly calamitous angle.10 An informed populace would be better positioned to rein in those who wish to abuse CRISPR in such a fashion. By understanding the problems CRISPR can address, we can also identify the problems CRISPR is ill-suited to handle. In the absence of popular consciousness and critique, companies and private entities will rush in to fill their pockets.
Because the Food and Drug Administration only monitors the safety of gene-edited foods if the manufacturer requests a consultation, Calyxt voluntarily submitted its soybean to the FDA for approval prior to its use in a restaurant chain in the Midwest. They did, and the oil made its way onto dinner tables by early 2019.11
However, it was Calyxt’s justification for their modified foods that was disturbing. At the time, Calyxt’s About Us page implied that declining life expectancies in America were attributable to soaring rates of diabetes and obesity. With fat-free foods, they might be able to do their part in reversing this worrying trend. Therefore, Calyxt declared, “it is unethical NOT to use our technologies to address these issues head-on.”12
Of course, Calyxt ignored the fact that social determinants have driven the drop in life expectancy in the US, not fatty foods. A study in 2015 stated that “death from diabetes has not been an increasing threat” and that obesity accounted for “only a small fraction” of “deterioration of midlife self-assessed health, mental health, reported pain.”13 A 2018 study of declining life expectancy in high income countries reaffirmed this point, stating that deaths in countries with declining life expectancy were concentrated among younger people (0–65 year olds). They attribute the declines to drugs and other external causes of death rather than diet.14 Calyxt has since updated their About Us page and makes no further mention of these claims.
A similar scenario played out in China. Dr. He Jiankui justified his CRISPR experiment on Lulu and Nana by framing it as an intervention to inoculate them against HIV by turning off their CCR5 gene.15 Increasing access to physical contraceptives like condoms through public health measures is an effective and less hazardous means of dealing with the problem. Similarly, if the concern is that an HIV+ parent might pass the virus along to their child, there are antiretroviral drugs that can be used to mitigate risk.
In contrast to these proven approaches, there are potentially alarming consequences to Dr. He’s dangerous, poorly executed experiment.16 As many scientists have indicated, we do not fully understand the effect of losing CCR5. It is the case that individuals with mutations in this gene are highly resistant to contracting HIV, but other health consequences these children might face remain unclear.17 We also do not know whether CCR5 is part of a larger genetic network` which might be disrupted if CCR5 is removed from the cell.18
Moreover, CRISPR can be imprecise. Issues with CRISPR’s specificity could mean that other parts of the genome are edited accidentally along with CCR5. These “off-target effects” have been shown in some contexts to lead to large rearrangements of DNA and even chromosomal losses, each of which could cause significant clinical pathologies down the line.19 Specificity may be improved with newer CRISPR technologies, but for the moment, and including Dr. He’s study, these issues remain a primary safety concern.
This idea of biological unknowns hearkens to an argument made by Hubbard in the pages of Science for the People in 1983 regarding embryonic genome manipulation.20 She stressed that our lack of understanding about genetics is concerning enough to avoid trying to alter human DNA altogether. Until these risks are addressed, Hubbard cautioned against trying to edit the genome of a living human being.
Even today, scientists have a deeply limited comprehension of the human genetic landscape. We are still in the dark about how much of our DNA works, and what the consequences will be of changing it. This is a limitation that may be alleviated as we learn more about genetics, but for the time being, there is an argument to be made that we simply don’t know enough to take the chance — especially when there are effective alternatives available to us.

Bridging the Education Gap
In 2019, the US National Academies of Medicine and Science and the UK Royal Society established an expert commission to make recommendations on the use of germline, or heritable, CRISPR genome editing. Part of their charge was to engage the community in sessions of public comment to help inform the ethical debate about editing DNA in humans. In their final report, the commission revealed that they received just 83 public responses.21 Whether this was due to poor advertising, an uninformed populace, or both, the message is clear that CRISPR researchers and regulators must do more to engage the public on this issue.
I and many of my colleagues have written about the desperate need to inform the public about what exactly CRISPR is, let alone its ethical implications.22 We face an uphill battle in some ways; CRISPR remains the boogeyman in everything from video games to television to film, often depicted as a bioterrorism threat with the capacity to create horrifying monsters. Consider the games Spider-Man and Deus Ex: Mankind Divided, where the antagonists wield CRISPR as a tool to bring society to its knees, or Rampage, a 2016 sci-fi film starring The Rock, where genome editing fuels the rapid growth of creatures that subsequently wreak havoc on the city. For many, these portrayals are quite possibly the first they’ve heard of CRISPR — if they’ve heard of CRISPR at all.
Without any real understanding of how CRISPR works, and thus its applications and limitations, it is hard to imagine engaging in an informed debate about the safety, ethics, and regulation of its use. Ironically, scaremongering about its potential as an agent of mass destruction crowds out the real concerns experts have about CRISPR, such as its safety and the possibility of the technology ushering in a new era of eugenics.23 Easily disprovable misinformation provides companies with an easy target for proving their bona fides. They can debunk fantastical “hype” and push their agenda without dealing with the messy side of the science.
To take an example of this phenomenon playing out in a different context, anti-vaccination voices have pushed pseudoscientific ideas about the dangers of vaccines.24 In response, governmental and corporate actors point to ample reserves of scientific research supporting their claims of efficiency and safety for their product. In doing so, pharmaceutical companies get to portray themselves as evidence-based and scientific in the face of irrationality. This positioning helps shield them from legitimate critiques of how private enterprise has bungled the development of vaccines in the past, or how major pharmaceutical companies have shown their willingness to price gouge, placing profit over human life.25 One can easily imagine these same companies pushing new CRISPR technologies in the same way, dismissing the conspiracy theory while obfuscating deeper issues with their new product.
Public education is also crucial because we, as scientists, are in need of outside perspective on our work. We are often untrained in ethical considerations of our research and tend to be demographically homogeneous relative to the population.26 We cannot hope to address the totality of the concerns around concepts like genome editing by ourselves. We need the input of laypeople, bioethicists, lawyers, artists, religious leaders, and philosophers. Our community has to consider the perspectives of disability rights advocates and racial justice organizers. If these groups, which are likely new to the biology of genome editing, are left out of the conversation or with misapprehensions about what CRISPR entails, scientists will be poorer for the loss of their points of view. And in the end, as we have seen time and again in medicine, the most vulnerable will suffer the consequences of decisions made in their absence.27
Marching Forward, with Trepidation
There are myriad opportunities for scientists to address these concerns around CRISPR. This is a technology that has already garnered a remarkable legacy: it has been used to successfully treat Duchenne muscular dystrophy, retinitis pigmentosa, tyrosinemia type I, hemophilia, cancer, sickle cell anemia, and many other diseases.28 Allowing these groundbreaking advances to disappear amid fearmongering misinformation would be a grave mistake. But it is also important to dispel the hype around CRISPR to highlight both the real promise and material concerns around the technology.
As my colleague Dr. Ayokunmi Ajetunmobi and I called for in 2017 along with many others, we must engage with the public using all available forms of communication — videos, podcasts, forums, public talks — to inform the public about the technologies at stake.29 We cannot be content with our published CRISPR papers, our academic presentations, and our popular science books. We have to engage with our communities and invite vulnerable stakeholders to the table and support them as they advocate for their needs. Neglecting to do so creates a vacuum that will inevitably be filled with anti-science opportunists preying on public emotion, just as we’ve seen with the anti-vaccination movement and climate denialists.
CRISPR biologists should also be vocal about our work being used irresponsibly. Every person performing CRISPR experiments in the lab is contributing to the body of knowledge around this emerging technology. Whether or not we directly participate in its more nefarious uses, we are providing the evidence necessary to refine CRISPR as a tool for genetic manipulation. This means that we are both responsible for its development and uniquely placed as experts to raise the alarm on these issues. As such, we should make it clear to our peers and the broader public that CRISPR is not a panacea for social ills, and be forceful about identifying the root causes of social decay, like racism, capitalism, and state repression, all of which cannot be addressed through genome editing.
Finally, in my field of human genetics, we can refuse to enable the research of individuals like Dr. He. Dr. Ajetunmobi and I laid out in a 2019 CRISPR Journal article a framework for an academic boycott of researchers carrying out dangerous germline editing experiments in viable embryos.30 The boycott rests on three concepts. First, researchers must withdraw all support, collaboration, and advice from scientists who they know to be engaging in germline editing research in viable embryos. Second, researchers should refrain from supporting complicit journals and conferences that host this kind of research. Finally, academics contacted by germline editing researchers should alert their colleagues and institutions immediately to help deprive offenders of resources. It should be noted that the National Academies study, released a year after our paper, suggested a similar reporting mechanism. Although the goal is similar, their model rests on reporting violators via a centralized, anonymized “service” which can liaise with “regulatory authorities,” while we recommend a more horizontalist approach of peer-to-peer communication without the involvement of the state.31
It takes time to make informed, evidence-based, and socially conscious recommendations on issues of this magnitude. Our proposed boycott may buy some time for the international community to do the necessary work of engaging the public democratically. This will, hopefully, provide us the space we need as we seek consensus on how and whether to proceed with the arguably lifesaving, potentially devastating new tool we call CRISPR.
February 6, 2021: An earlier version of this article suggested Calyxt did not seek FDA approval for its soybean oil, based on reporting by The Washington Post. This was in error, as indicated by this FDA letter.
Infographic Citations
- Eva Sirinathsinghji, “Gene Drive Organisms,” African Centre for Biodiversity, May 2019, https://web.archive.org/web/20200718055652/https://www.acbio.org.za/en/gene-drive-organisms-what-africa-should-know-about-actors-motives-and-threats-biodiversity-and-food.
- Charlotte Douglas et al., “Generating Single-Sex Litters: Development of CRISPR-Cas9 Genetic Tools to Produce All-Male Offspring,” bioRxiv (September 2020), https://doi.org/10.1101/2020.09.07.285536.
- Anne Finger, “Claiming All of Our Bodies: Reproductive Rights and Disability,” in Test-tube Women: What Future for Motherhood?, ed. Renate Klein, Rita Arditti, Shelley Minden (London, Boston and Melbourne: Pandora Press, 1984), 281–297.
References
- “Press release: The Nobel Prize in Chemistry 2020,” The Nobel Foundation, October 7, 2020, https://www.nobelprize.org/prizes/chemistry/2020/press-release/.
- Nathaniel Comfort, “Genetic Determinism Rides Again,” Nature, 561 (2018): 461–463, https://doi.org/10.1038/d41586-018-06784-5.
- Michael Marmot et al., “Fair Society, Health Live (The Marmot Review): Executive Summary,” Institute of Health Equity (2010): 11, http://www.instituteofhealthequity.org/resources-reports/fair-society-healthy-lives-the-marmot-review/fair-society-healthy-lives-exec-summary-pdf.pdf.
- Kenneth D. Kochanek, Sherry L. Murphy, Jiaquan Xu, and Elizabeth Arias.“Deaths: Final Data for 2017,” National Vital Statistics Reports, 68, no. 9 (2019): 1–6, https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_09-508.pdf.
- Gustavo Turecki, David A. Brent, “Suicide and Suicidal Behaviour,” The Lancet, 387, no. 10024 (2016): 1227–1239, https://doi.org/10.1016/S0140-6736(15)00234-2.
- John A. Kaufman, Leslie K. Salas-Hernández, Kelli A. Komro, and Melvin D. Livingston, “Effects of Increased Minimum Wages by Unemployment Rate on Suicide in the USA,” BMJ, 74, no. 3 (2016): 219–224. http://dx.doi.org/10.1136/jech-2019-212981.
- President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research, “Splicing Life: A Report on the Social and Ethical Issues of Genetic Engineering with Human Beings,” National Information Resource on Ethics and Human Genetics, 1982, https://bioethics.georgetown.edu/documents/pcemr/splicinglife.pdf.
- Alyssa Botelho, “The Insights of Radical Science in the CRISPR Gene-Editing Era: A History of Science for the People and the Cambridge Recombinant DNA Controversy,” Science as Culture, (2019): 1–30, https://doi.org/10.1080/09505431.2019.1623190.
- Ruth Hubbard, “Human Embryo and Gene Manipulation,” Science for the People, 15 no. 3 (1983): 24–27, http://science-for-the-people.org/wp-content/uploads/2015/07/SftPv15n3s.pdf.
- Rob Stein, “Scientists Release Controversial Genetically Modified Mosquitoes In High-Security Lab,” NPR, February 20, 2019, https://www.npr.org/sections/goatsandsoda/2019/02/20/693735499/scientists-release-controversial-genetically-modified-mosquitoes-in-high-security.
- Dan Charles, “Will Gene-Edited Food Be Government Regulated?” NPR, May 10, 2019, https://www.npr.org/sections/thesalt/2019/05/10/717273970/will-gene-edited-food-be-government-regulated.
- “Mission,” Calyxt, archived August 3, 2018, https://web.archive.org/web/20180803012901/https://calyxt.com/about-us/.
- Anne Case and Angus Deaton, “Rising Morbidity and Mortality in Midlife Among White Non-Hispanic Americans in the 21st Century,” PNAS, 112, no. 49 (2015): 15078–15083, https://doi.org/10.1073/pnas.1518393112.
- Jessica Y. Ho and Arun S. Hendi, “Recent Trends in Life Expectancy Across High Income Countries: Retrospective Observational Study,” BMJ, 362 (2018), https://doi.org/10.1136/bmj.k2562.
- Antonio Regalado, “Chinese Scientists Are Creating CRISPR Babies,” MIT Technology Review, November 25, 2018, https://www.technologyreview.com/2018/11/25/138962/exclusive-chinese-scientists-are-creating-crispr-babies/.
- Sharon Begley, “The ‘CRISPR Babies’ Experiment Was More Flawed Than Scientists First Realized,” STAT, December 5, 2018, https://www.statnews.com/2018/12/05/crispr-babies-experiment-more-flawed-than-first-realized/.
- David Cyranoski, “Baby Gene Edits Could Affect a Range of Traits,” Nature News, December 12, 2018, https://doi.org/10.1038/d41586-018-07713-2.
- Yong Xie, Shaohua Zhan, Wei Ge, and Peifu Tang, “The Potential Risks of C-C Chemokine Receptor 5-Edited Babies in Bone Development,” Bone Research, 7, no. 4 (2019), https://www.nature.com/articles/s41413-019-0044-0
- Michael Kosicki, Kärt Tomberg, and Allan Bradley, “Repair of Double-Strand Breaks Induced by CRISPR–Cas9 Leads to Large Deletions and Complex Rearrangements,” Nature Biotechnology, 36, no. 8 (2018): 765–771, https://doi.org/10.1038/nbt.4192; Chongwei Bi et al., “Long-Read Individual-Molecule Sequencing Reveals CRISPR-Induced Genetic Heterogeneity in Human ESCs,” Genome Biology, 21, no. 213 (2020), https://doi.org/10.5281/zenodo.3977107; Ida Höijer et al., “Amplification-Free Long Read Sequencing Reveals Unforeseen CRISPR-Cas9 Off-Target Activity,” bioRxiv, (2020), https://doi.org/10.1101/2020.02.09.940486; Katherine J. Wu, “Crispr Gene Editing Can Cause Unwanted Changes in Human Embryos, Study Finds,” The New York Times, October 31, 2020, https://www.nytimes.com/2020/10/31/health/crispr-genetics-embryos.html.
- Hubbard, “Human Embryo.”
- National Academy of Medicine, National Academy of Sciences, and the Royal Society, “Heritable Human Genome Editing,” The National Academies Press, 2020, https://doi.org/10.17226/25665.
- Soren Hough and Ayokunmi Ajetunmobi. “The Future of CRISPR Applications in the Lab, the Clinic and Society,” in Precision Medicine, CRISPR, and Genome Engineering, ed. Stephen H. Tsang (Cham: Springer International Publishing, 2017), 157–78 https://doi.org/10.1007/978-3-319-63904-8_9.
- Teresa Watanabe, “UC Berkeley Is Disavowing Its Eugenic Research Fund After Bioethicist and Other Faculty Call it Out,” Los Angeles Times, October 26, 2020, https://www.latimes.com/california/story/2020-10-26/uc-berkeley-disavows-eugenics-research-fund.
- Dina Fine Maron, “Fact or Fiction?: Vaccines Are Dangerous,” Scientific American, March 6, 2015, https://www.scientificamerican.com/article/fact-or-fiction-vaccines-are-dangerous/.
- Vanessa A. Bee, “Would We Have Already Had a COVID-19 Vaccine Under Socialism?” In These Times, April 20, 2020, https://inthesetimes.com/features/covid-19-coronavirus-vaccine-capitalism-socialism-innovation.html; Chris McGreal and Jessica Glenza, “How Price-Gouging of Opioid Overdose Cure Costs Lives: ‘There’s Never Enough,’” The Guardian, December 16, 2016, https://www.theguardian.com/society/2016/dec/16/opioid-overdose-naloxone-pharmaceutical-price-gouging; Jan Hoffman, “Purdue Pharma Tentatively Settles Thousands of Opioid Cases,” The New York Times, September 11, 2019, https://www.nytimes.com/2019/09/11/health/purdue-pharma-opioids-settlement.html.
- Cary Funk and Kim Parker, “Diversity in the STEM Workforce Varies Widely Across Jobs,” Pew Research Center, January 9, 2018, https://www.pewsocialtrends.org/2018/01/09/diversity-in-the-stem-workforce-varies-widely-across-jobs/.
- Harry Kretchmer, “A Brief History of Racism in Healthcare,” World Economic Forum, July 23, 2020, https://www.weforum.org/agenda/2020/07/medical-racism-history-covid-19/; Elisabeth Mahase, “Black Babies Are Less Likely to Die When Cared for by Black Doctors, US Study Finds,” BMJ, 370 (2020), https://doi.org/10.1136/bmj.m3315.
- Leonela Amoasii et al., “Gene Editing Restores Dystrophin Expression in a Canine Model of Duchenne Muscular Dystrophy,” Science, 362, no. 6410 (2018): 86–91, http://doi.org/10.1126/science.aau1549; Christopher E. Nelson et al., “Long-Term Evaluation Of AAV-CRISPR Genome Editing for Duchenne Muscular Dystrophy,” Nature Medicine, 25 (2019): 427–432, https://doi.org/10.1038/s41591-019-0344-3; Yi-Ting Tsai et al., “Clustered Regularly Interspaced Short Palindromic Repeats-Based Genome Surgery for the Treatment of Autosomal Dominant Retinitis Pigmentosa,” Ophthalmology, 125, no. 9 (2018): 1421–1430, https://doi.org/10.1016/j.ophtha.2018.04.001; Hao Yin et al., “Genome Editing with Cas9 in Adult Mice Corrects a Disease Mutation and Phenotype,” Nature Biotechnology, 32 (2014), https://doi.org/10.1038/nbt.2884; Hainan Chen, “Hemophilia A Ameliorated in Mice by CRISPR-Based In Vivo Genome Editing of Human Factor VIII,” Scientific Reports, 9, no. 16838 (2019), https://doi.org/10.1038/s41598-019-53198-y; Edward A. Stadtmauer et al., “CRISPR-Engineered T Cells in Patients with Refractory Cancer,” Science, 367, no. 6481 (2020), http://doi.org/10.1126/science.aba7365; Rob Stein, “In A 1st, Doctors In U.S. Use CRISPR Tool To Treat Patient With Genetic Disorder,” NPR, July 29, 2019, https://www.npr.org/sections/health-shots/2019/07/29/744826505/sickle-cell-patient-reveals-why-she-is-volunteering-for-landmark-gene-editing-st.
- Hough, “The Future.”
- Soren Hough and Ayokunmi Ajetunmobi, “A CRISPR Moratorium Isn’t Enough: We Need a Boycott,” The CRISPR Journal, 2, no. 6 (2019): 343–345, http://doi.org/10.1089/crispr.2019.0041.
- National Academy, “Heritable Human.”